How the safeCircle® Pooled Testing Program Works
To learn more about Applied DNA Clinical Labs’ COVID-19 Pooled Testing Program, call 631.901.4264 or submit your information below:
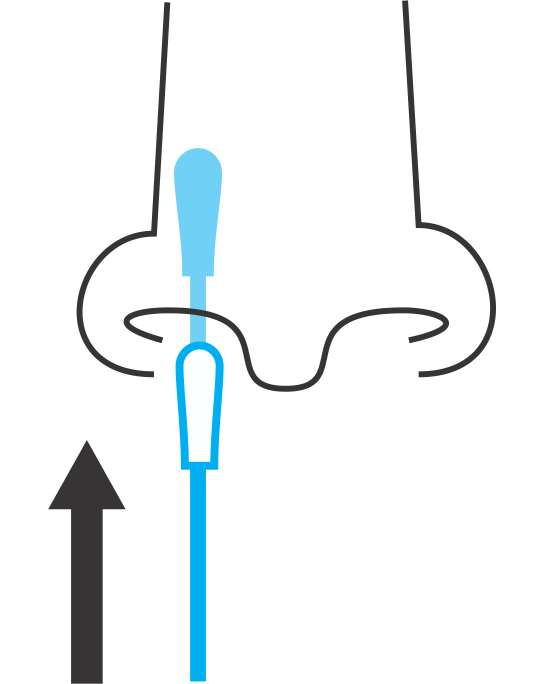
Sample Collection
- Short, non-invasive anterior nasal swab (ANS) samples are gathered in a central location such as a workplace or school with easy-to-use collection kits provided by ADCL.
Transporting Samples
- Sample transport options are arranged within service level agreements established with each client. Options include:
- Courier service: ADCL can arrange for a courier service to pick up and transport samples to our lab for testing.
- Overnight package shipment: Using provided overnight shipping materials, samples are transported to ADCL for testing.
- The safeCircle program can accommodate special transportation arrangements depending on unique client needs.
Testing
- Once samples are received at ADCL’s laboratory, the samples are pooled into 5-sample pools for testing. Pooling allows ADCL to test more samples with fewer testing materials and achieve faster turnaround times.
- Each 5-sample pool is tested with the EUA-Authorized RT-PCR Linea™ COVID-19 SARS-CoV-2 Assay Kit.
- If a positive pool is detected, all samples contained in the positive pool are individually diagnostically reflex tested with the Linea™ COVID-19 SARS-CoV-2 Assay Kit to identify the infected individual.
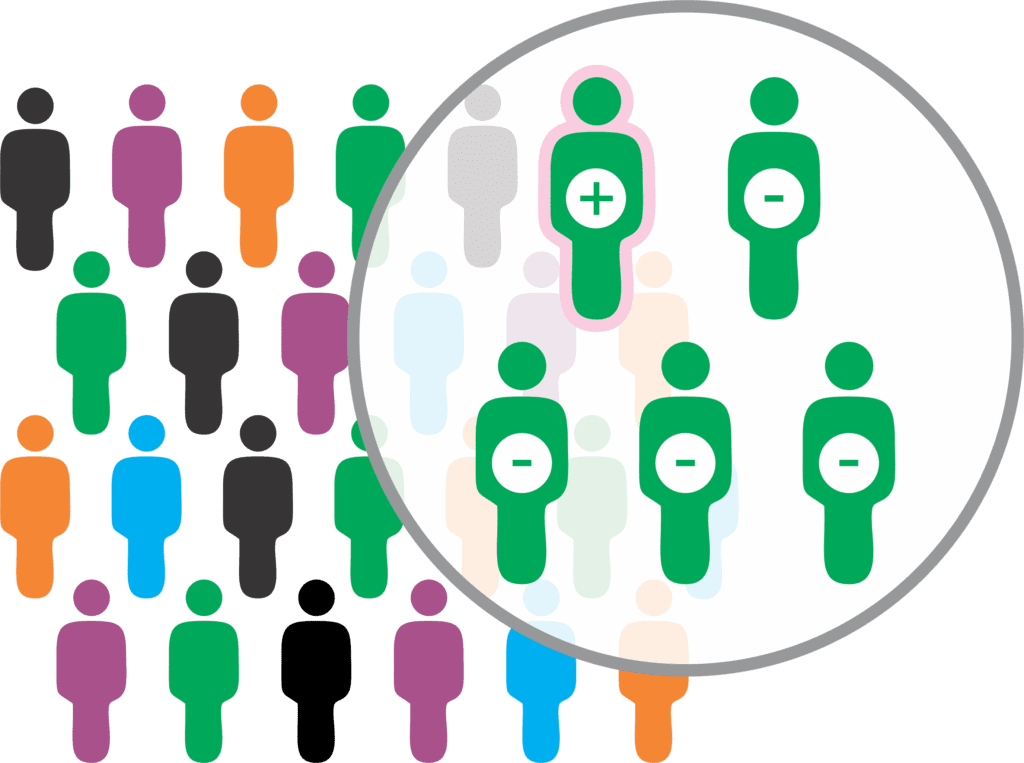
Results Reporting — Pooled Screening Testing
- If a pool tests negative, all individuals who provided the samples contained within the negative pool can be presumed negative for SARS-CoV-2 infection.
- If a pool tests positive, ADCL will perform individual diagnostic reflex testing on each sample contained in the pool to identify the positive individual. All individuals who provided samples that tested negative via individual diagnostic reflex testing will be reported as negative.
- Turnaround time is typically 24 hours from sample receipt at ADCL’s laboratories.
Results Reporting — Pooled Surveillance Testing
- In accordance with the current surveillance testing guidances, the results of all samples tested under a surveillance program will be reported only in the aggregate to the client. No individual testing results are reported to the client, participants or any third-party. If one or more positive pools are identified by the program, the client’s program manager should refer the participants in the positive pools to their healthcare provider for potential individual diagnostic testing.
- Turnaround time is typically 24 hours from sample receipt at ADCL’s laboratories.
All testing is performed by Applied DNA Clinical Labs, LLC, 25 Health Sciences Drive, Suite 120, Stony Brook, NY 11790. PFI: 9546 CLIA: 33D2187353

