
APDN to Establish N. America’s 1st Enzymatic Large-Scale cGMP DNA Manufacturing Capacity in NY
October 24th, 2022

APDN to Establish N. America’s 1st Enzymatic Large-Scale cGMP DNA Manufacturing Capacity in NY
October 24th, 2022

APDN Receives Largest Single Purchase Order for LinearDNA To Date
October 10th, 2022

Manuscript Demonstrates that LinearDNA™ Vaccine Candidate was Protective in Virus Challenge Trial
September 30th, 2022

APDN, Cornell University College of Veterinary Medicine Research Collaboration
September 8th, 2022

APDN to Present at H.C. Wainwright 24th Annual Global Investment Conference
September 7th, 2022
Applied DNA Launches Monkeypox Testing Service
September 6th, 2022
APDN Submits PCR-based Monkeypox Diagnostic Test to NYS Dept of Health
August 19th, 2022

Applied DNA Reports Third Quarter Fiscal 2022 Financial Results
August 11th, 2022
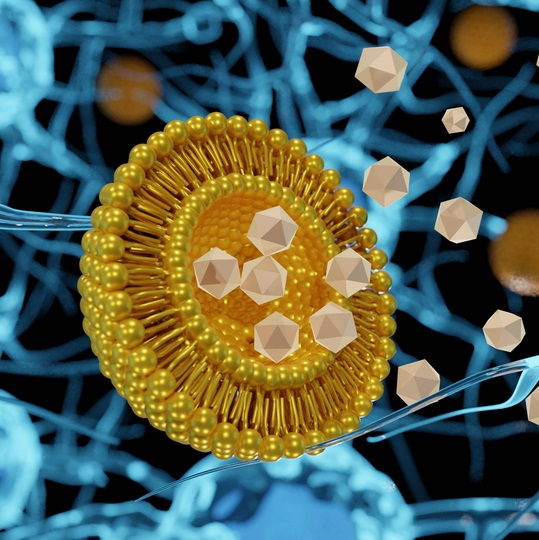
First Successful Administration of LinearDNA via Lipid Nanoparticles
August 10th, 2022

Our new ticker symbol is ‘BNBX’. Learn more here.