Linea™IVT — Better mRNA…Faster
A Combination of 2 Next Generation Technologies
LineaIVT is the combination of LRx’s enzymatically produced LinearDNA IVT templates and its proprietary Linea RNA polymerase. The unique combination redefined mRNA production, resulting in:
A simplified mRNA workflow
Conventional IVT mRNA Production
Linea™ IVT mRNA Production
With significantly Reduced or Eliminated dsRNA Impurities
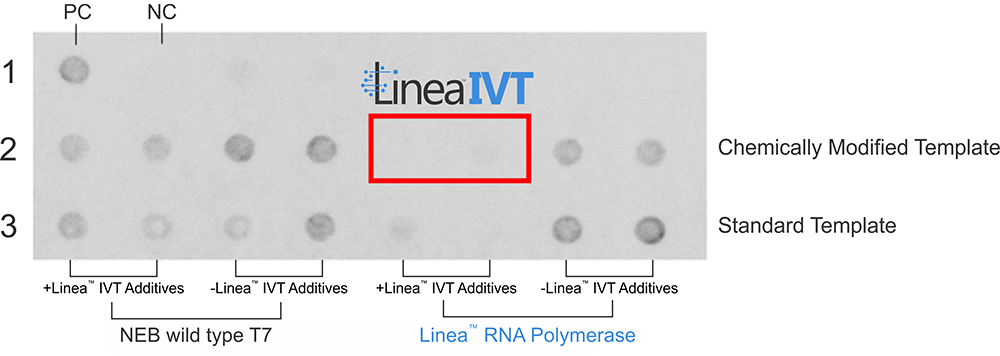
Without Sacrificing Yield
linDNA offers key advantages compared to pDNA:
Attribute
LinearDNA
Plasmid DNA
Risk of Antibiotic Resistance Gene Transfer
Endotoxin Risk
Cellular Purification Necessary
Know/Fixed Yield
Unwanted DNA in Final Product
Manufacturing Timeframe
Days
Weeks/Months
DNA Construct Optimization via Primer Modification
Able to Produce Long Homogenous Poly-A Tails (necessary for mRNA templates)
Percentage of Produced DNA Comprised of Target Therapeutic DNA Sequence
100%
≈ 40%
